250,000 people have tried 5Strands!
BEST SELLERS
4 EASY STEPS TO TAKE
-
![]()
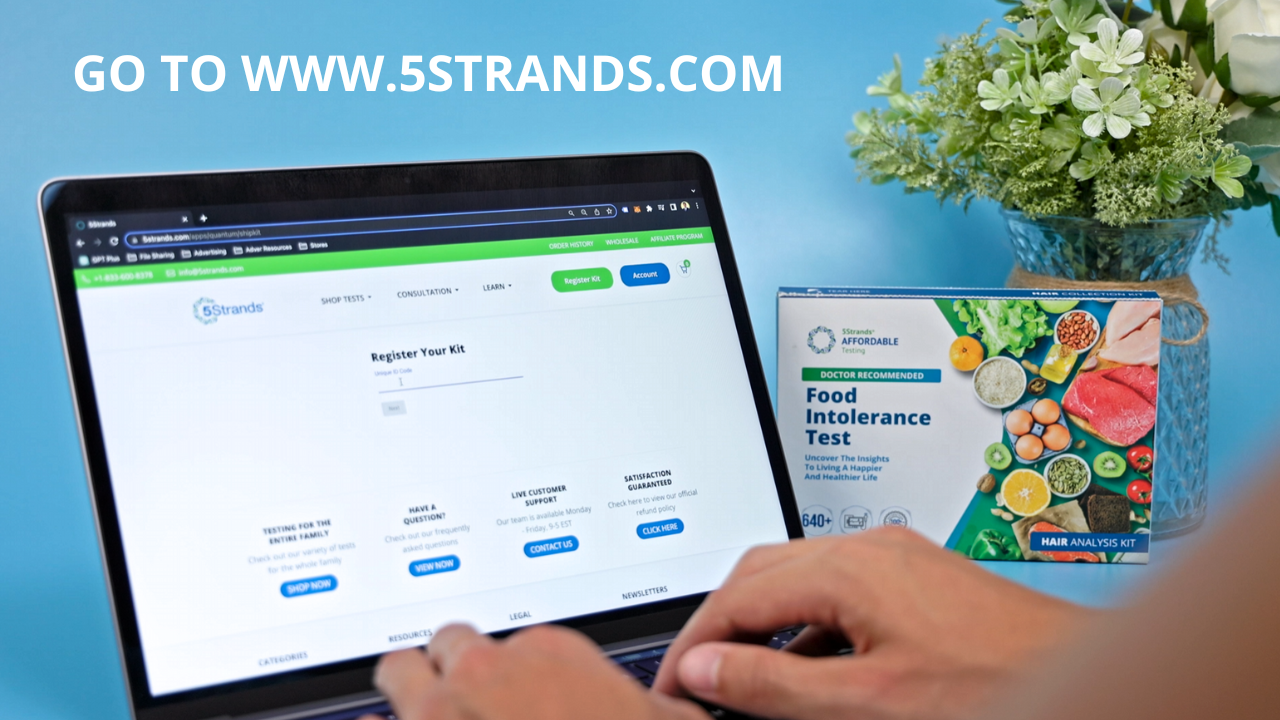
REGISTER KIT
Login to 5Strands.com and click REGISTER KIT. Use unique ID code provided inside kit.

-
![]()
COLLECT HAIR
Collect 5Strands of hair from head, arm or body and place it inside the provided bag.

-
![]()
MAIL SAMPLE
Place bag back inside the kit. Make sure postage is good to ship box back to us.

-
![]()
RECEIVE RESULTS
A week after we receive the kit, we will be able to send the results.
A ROAD MAP TO BETTER HEALTH
FREQUENTLY ASKED QUESTIONS
5Strands tests are available to residents in all 50 states. International shipping options are also available. We accept PayPal, Visa, MasterCard, Discover, and American Express, but feel free to contact us for other payment options.
Yes, 5Strands is able to provide services to any adult, child, dog, cat, horse, or other animals with hair. We have ran scans for infants as young as 2 month and fur babies as young as 1 month.
Collecting a hair sample is easy and non-invasive. Hair testing eliminates storage, transportation barriers, and usability constraints that are associated with other types of tissue samples. Hair is stable. It’s not affected by factors such as recent meals, stress, medications, etc.
Once the hair sample has been received at the processing center and logged into the system, it will be immediately sent for testing. The results take 5-7 days from the date it is received. Once the results have been completed, they will automatically be uploaded and available on the portal and your 5Strands app. You will also receive an emailed copy of the testing results to the email you registered with.
Once 5Strands has completed testing, your sample is stored in a container for up to 45 days. This allows opportunity for additional testing without sending a new sample. A biohazard company comes every 45 days to pick up the samples and disposes of them according to EPA, OSHA, and CDC guidelines.
Trusted By








WHAT DO OUR CUSTOMERS SAY?
INTOLERANCE VS ALLERGY

What is an intolerance?
An intolerance is a non-immunological response where the immune system is not activated.
What causes an intolerance?
An intolerance occurs when the body has difficulty digesting or breaking down certain foods, possibly due to enzyme deficiencies and overall gastrointestinal health.
What removes the intolerance?
After identifying a food or environmental intolerance, temporarily removing the item from your dietary and lifestyle exposure may reduce your severity of response upon future exposures.
What is an allergy?
A food allergy is when the immune system produces immunoglobulin E (IgE) antibodies in response to specific foods or item exposure.
What are the symptoms?
Allergic response symptoms include swelling, hives, rash, itching skin, swelling of the throat, breathing difficulties, and vomiting.
What are common food allergies?
The most common IgE-associated food allergies include milk, eggs, wheat, peanuts, nuts, fish, and sesame. 5Strands does NOT test for allergies, which are often permanent.

Give Your Pet A Voice About Their Diet & Lifestyle
Featured On






































